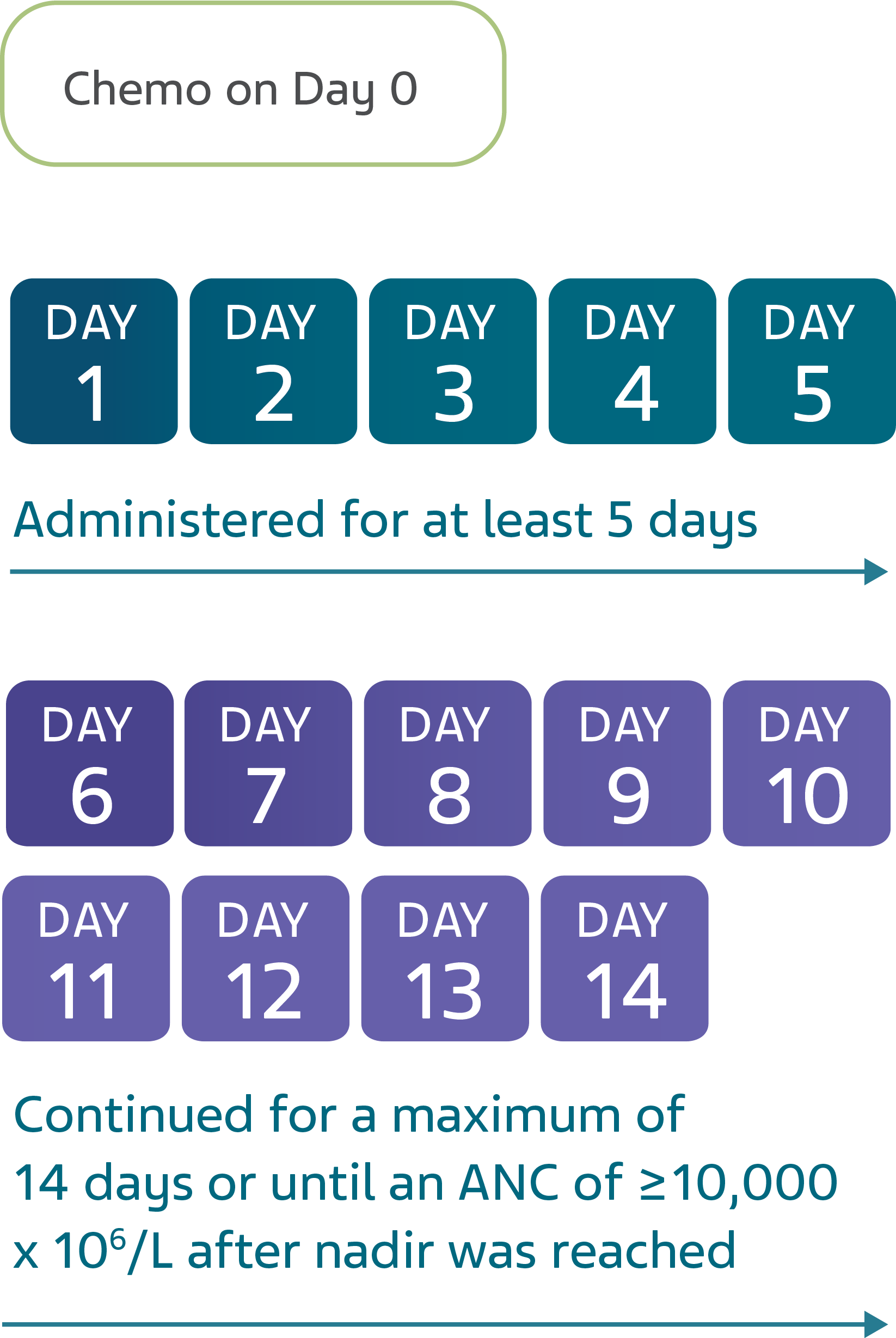
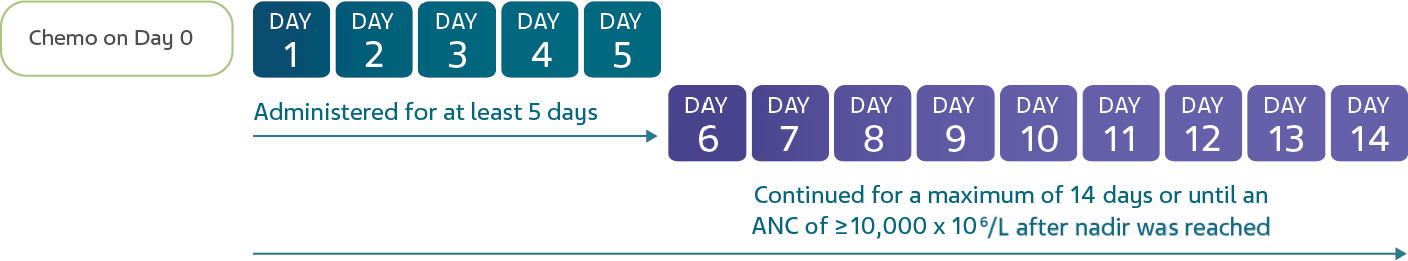
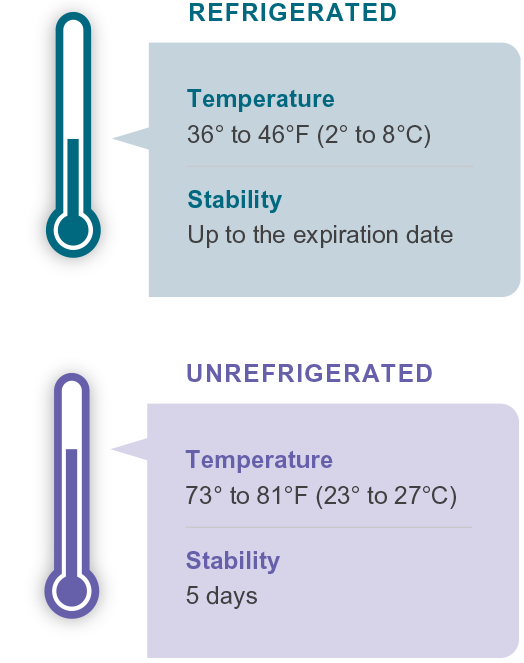
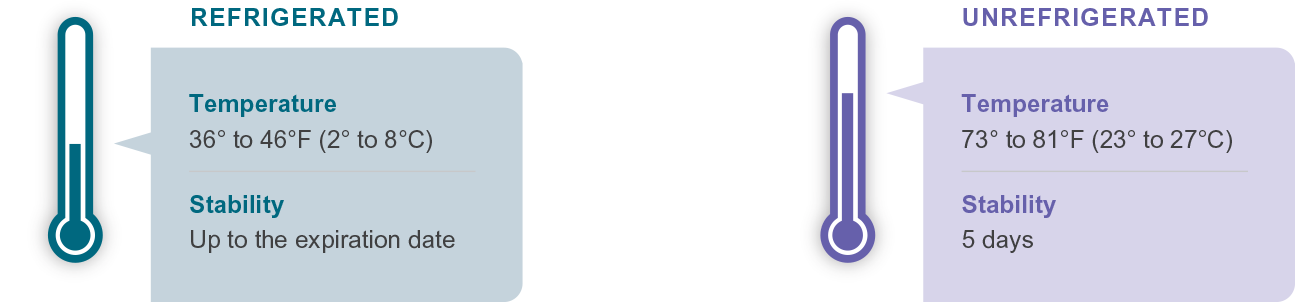
GRANIX is indicated to reduce the duration of severe neutropenia in adults and pediatric patients 1 month and older with non-myeloid malignancies receiving myelosuppressive anticancer drugs associated with a clinically significant incidence of febrile neutropenia.
Important Safety Information
-
Contraindication: GRANIX is contraindicated in patients with a history of serious allergic reactions to filgrastim or pegfilgrastim products.
-
Splenic Rupture: Splenic rupture, including fatal cases, can occur following the administration of filgrastim products. Evaluate patients who report upper abdominal or shoulder pain for an enlarged spleen or splenic rupture. Discontinue GRANIX if splenic rupture is suspected or confirmed.
-
Acute respiratory distress syndrome (ARDS): ARDS can occur in patients receiving filgrastim products. Evaluate patients who develop fever and lung infiltrates or respiratory distress after receiving GRANIX, for ARDS. Discontinue GRANIX in patients with ARDS.
-
Serious Allergic reactions: Serious allergic reactions, including anaphylaxis, can occur in patients receiving GRANIX. Reactions can occur on initial exposure. The administration of antihistamines‚ steroids‚ bronchodilators‚ and/or epinephrine may reduce the severity. Permanently discontinue GRANIX in patients with serious allergic reactions. Do not administer GRANIX to patients with a history of serious allergic reactions to filgrastim or pegfilgrastim.
-
Sickle Cell Disorders: Severe and sometimes fatal sickle cell crises can occur in patients with sickle cell disorders receiving filgrastim products. Discontinue GRANIX if sickle cell crisis occurs.
-
Glomerulonephritis: Glomerulonephritis can occur in patients receiving filgrastim products. The diagnoses were based on azotemia, hematuria (microscopic and macroscopic), proteinuria, and renal biopsy. Generally, events of glomerulonephritis resolved after dose reduction or discontinuation of the filgrastim product. If glomerulonephritis is suspected, evaluate for cause. If causality is likely, consider dose-reduction or interruption of GRANIX.
-
Myelodysplastic Syndrome(MDS) and Acute Myeloid Leukemia (AML)
-
Patients with Severe Chronic Neutropenia (SCN)
-
Confirm the diagnosis of SCN before initiating GRANIX therapy
-
MDS and AML have been reported to occur in the natural history of congenital neutropenia without cytokine therapy
-
Cytogenetic abnormalities, transformation to MDS, and AML have also been observed in patients treated with GRANIX for SCN
-
Based on available data including a postmarketing surveillance study, the risk of developing MDS and AML appears to be confined to the subset of patients with congenital neutropenia.
-
Abnormal cytogenetics and MDS have been associated with the eventual development of myeloid leukemia
-
The effect of GRANIX on the development of abnormal cytogenetics and the effect of continued GRANIX administration in patients with abnormal cytogenetics or MDS are unknown. Monitor patients for signs and symptoms of MDS/AML in these settings.
-
If a patient with SCN develops abnormal cytogenetics or myelodysplasia‚ the risks and benefits of continuing GRANIX should be carefully considered
-
-
Patients with Breast and Lung Cancer
-
MDS and AML have been associated with the use of GRANIX in conjunction with chemotherapy and/or radiotherapy in patients with breast and lung cancer. Monitor patients for signs and symptoms of MDS/AML in these settings.
-
-
-
Capillary leak syndrome (CLS): CLS can occur in patients receiving filgrastim products and is characterized by hypotension, hypoalbuminemia, edema and hemoconcentration. Episodes vary in frequency, severity and may be life-threatening if treatment is delayed. Patients who develop symptoms of CLS should be closely monitored and receive standard symptomatic treatment, which may include a need for intensive care.
-
Potential for tumor growth stimulatory effects on malignant cells: The granulocyte colony-stimulating factor (G-CSF) receptor, through which GRANIX acts, has been found on tumor cell lines. The possibility that GRANIX acts as a growth factor for any tumor type, including myeloid malignancies and myelodysplasia, diseases for which GRANIX is not approved, cannot be excluded.
-
Leukocytosis: White blood cell counts of 100‚000/mm3 or greater were observed in approximately 2% of patients receiving filgrastim products at dosages above 5 mcg/kg/day. In patients with cancer receiving GRANIX as an adjunct to myelosuppressive chemotherapy‚ to avoid the potential risks of excessive leukocytosis‚ it is recommended that GRANIX therapy be discontinued if the ANC surpasses 10‚000/mm3 after the chemotherapy-induced ANC nadir has occurred. Monitor CBCs at least twice weekly during therapy. Dosages of GRANIX that increase the ANC beyond 10‚000/mm3 may not result in any additional clinical benefit. In patients with cancer receiving myelosuppressive chemotherapy‚ discontinuation of filgrastim products therapy usually resulted in a 50% decrease in circulating neutrophils within 1 to 2 days‚ with a return to pretreatment levels in 1 to 7 days.
-
Simultaneous Use with Chemotherapy and Radiation Therapy Not Recommended: The safety and efficacy of filgrastim products, including GRANIX, given simultaneously with cytotoxic chemotherapy have not been established. Because of the potential sensitivity of rapidly dividing myeloid cells to cytotoxic chemotherapy‚ do not use GRANIX in the period 24 hours before through 24 hours after the administration of cytotoxic chemotherapy. The safety and efficacy of GRANIX have not been evaluated in patients receiving concurrent radiation therapy. Avoid the simultaneous use of GRANIX with chemotherapy and radiation therapy.
-
Nuclear Imaging: Increased hematopoietic activity of the bone marrow in response to growth factor therapy has been associated with transient positive bone-imaging changes. Consider this when interpreting bone-imaging results.
-
Aortitis: Aortitis has been reported in patients receiving another filgrastim product. It may occur as early as the first week after start of therapy. Manifestations may include generalized signs and symptoms such as fever, abdominal pain, malaise, back pain, and increased inflammatory markers (e.g., c reactive protein and white blood cell count). Consider aortitis in patients who develop these signs and symptoms without known etiology. Discontinue GRANIX if aortitis is suspected.
-
Alveolar Hemorrhage:Alveolar hemorrhage manifesting as pulmonary infiltrates and hemoptysis requiring hospitalization has been reported in healthy donors undergoing peripheral blood progenitor cell (PBPC) collection treated with another filgrastim product. Hemoptysis resolved with discontinuation of filgrastim. The use of GRANIX for PBPC mobilization in healthy donors is not an approved indication.
-
Most common treatment-emergent adverse reaction: Most common adverse reaction (≥1%) to GRANIX is bone pain.
-
The Most common side effects of GRANIX in children include:
- decreased platelet count
- headache
- fever
- diarrhea
- pain in the arms and legs
Please see Full Prescribing Information.